Impact of ten-valent pneumococcal conjugate vaccine in Finland
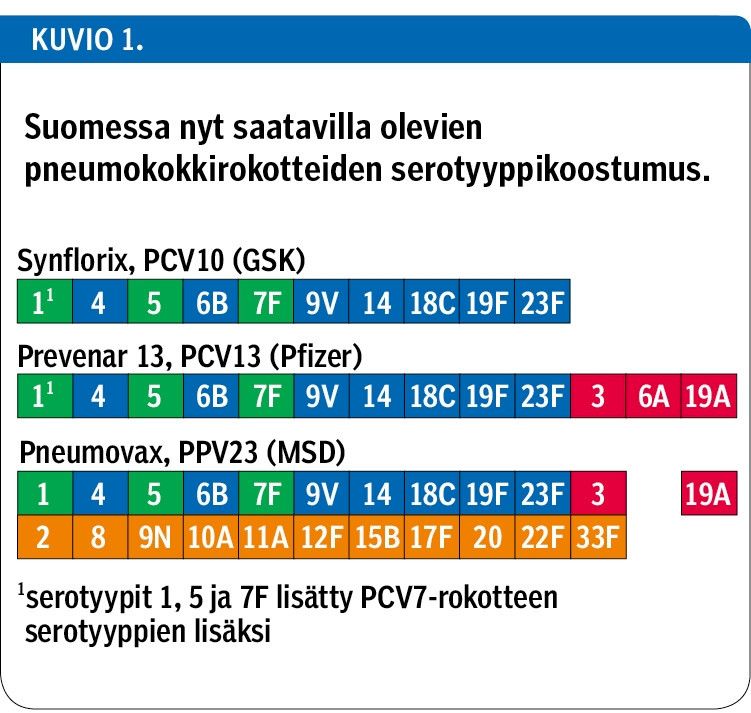
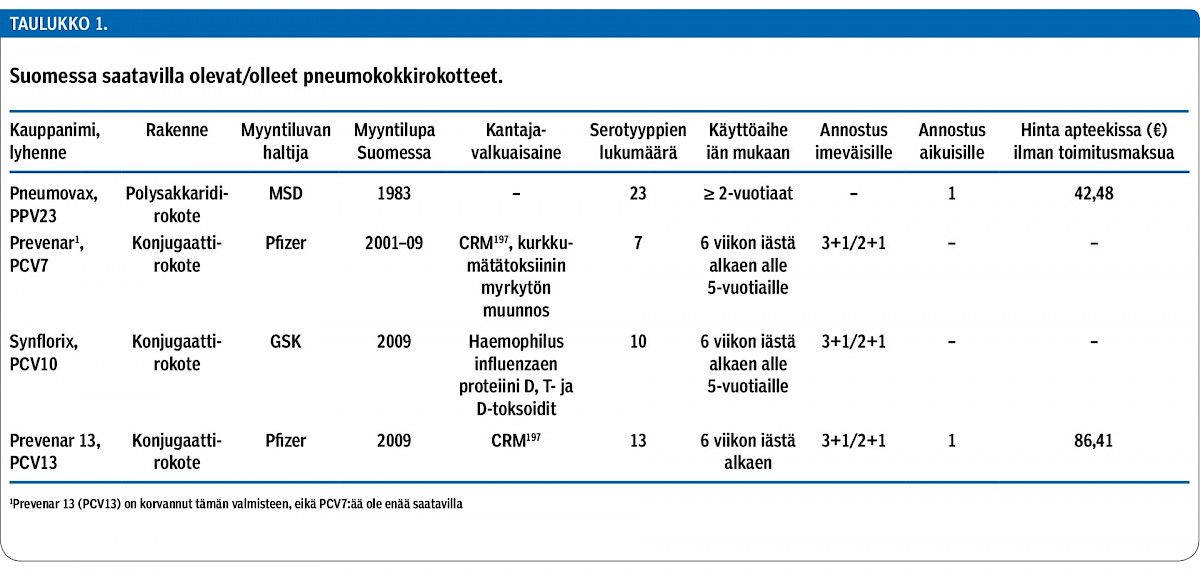
Streptococcus pneumoniae causes severe invasive pneumococcal disease (IPD, e.g. meningitis and sepsis) and pneumonia, but also mild, yet common, upper respiratory tract infections such as otitis media in children and sinusitis in adults. Pneumococcal vaccines have been developed to reduce these diseases. The polysaccharide vaccine (Pneumovax, MSD) with 23 serotypes is poorly immunogenic in infants. Therefore, pneumococcal conjugate vaccines (PCV) were developed, and currently there are two vaccines available, a 10-valent vaccine (PCV10, Synflorix, GSK) and a 13-valent vaccine (PCV13, Prevenar 13, Pfizer) which is a successor of the first-developed 7-valent PCV.
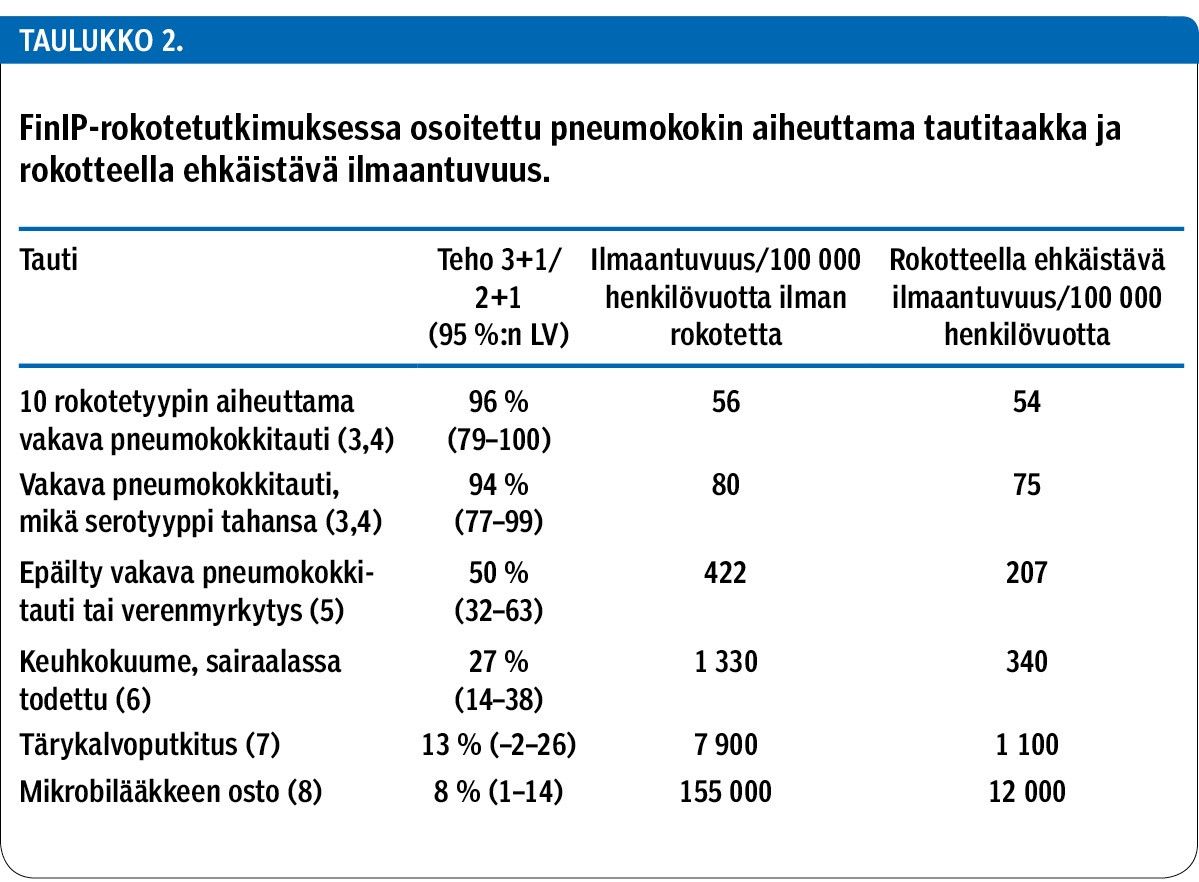
PCV10 was investigated in the Finnish Invasive Pneumococcal disease (FinIP) vaccine effectiveness trial prior to introduction into the National Vaccination Programme (NVP) in 2010 using a 2+1 schedule. PCV10 effectiveness and impact have been thoroughly documented against a variety of disease outcomes. In addition to a remarkable reduction in infant IPD, a reduction in pneumonia, tympanostomy placement and antimicrobial consumption has also been shown both in the FinIP trial setting and after NVP introduction on the basis of the nation-wide health registers. Additionally, for the first time globally, the effectiveness of the PCV was documented against clinically suspected IPD, a disease syndrome similar to laboratory-confirmed IPD, but with the laboratory evidence missing. On the basis of hospital discharge register data on clinical ICD-10 diagnoses compatible with IPD or unspecified sepsis, the pneumococcal severe disease burden was estimated to be many times that of laboratory-confirmed IPD only.
Since introduction of the NVP, an indirect impact (i.e. herd effect) has been observed in unvaccinated children, adults and the elderly. Introduction of pneumococcal vaccines into the NVP for the elderly is not considered cost-effective on the basis of the current evidence.